


























Our eyes act as intermediaries between the electromagnetic radiation all around us and our brains. In order to "see" something our eyes must convert visible light into a language the brain can understand. In this case, light cues must somehow be converted into the bioelectric pulses (called action potentials) that the brain can decode. The process of converting external stimuli into bioelectric signals is called sensory transduction. In the human eye, the transduction of light begins when a photon is absorbed by the photopigment rhodopsin in rods or by one of the three different photopigments found in the cones. Since rhodopsin is a ubiquitous light-sensing molecule in nature, we will focus our discussion on how it works.
 |
|
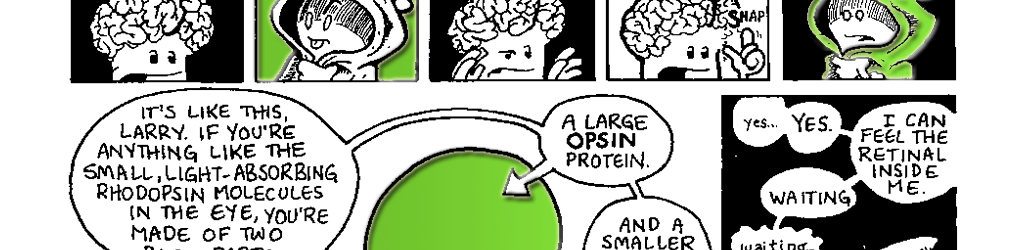
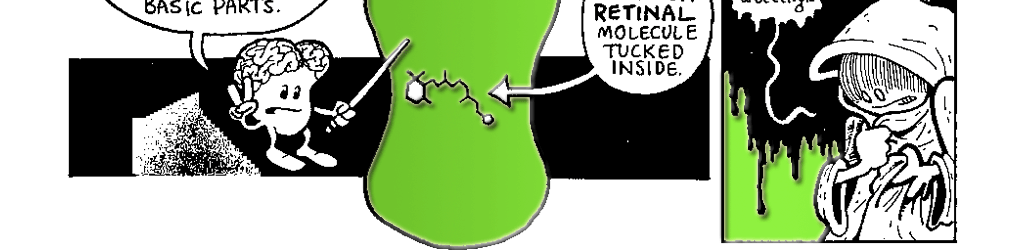
RhodopsinA molecule of rhodopsin is composed of two parts: a large opsin protein and a smaller retinal molecule. Like all proteins, the opsin is composed of amino acids. Those amino acids are strung together in a specific sequence called the protein’s primary structure (Fig. 7.1 A). In the case of bovine rhodopsin, the primary structure of the opsin is a string of 348 amino acids. But proteins don’t exist as nice neat strings. Once a protein’s amino acids are assembled, they begin to interact with each other in specific ways, and these interactions cause the long protein string to start folding up on itself. A protein folds in many ways. There can be small regional changes in a protein that only affect a handful of the amino acids. These cause some parts of the protein to coil into a spring-like alphahelix or perhaps fold into a corrugated beta-pleated sheet. These regional changes in a protein are called the secondary structure (Fig. 7.IB). Eventually, the entire protein, with all its secondary structures, folds in on itself. This overall shape of the protein is called its tertiary structure (Fig. 7.1C). In some cases, a protein joins with other proteins to form a larger protein molecule (sort of like how Moe, Larry and Curly came together to form the Three Stooges). In this case, each separate protein is considered a subunit of the larger protein. The shape of this amalgam of protein subunits is referred to as the protein’s quaternary structure (Fig. 7.ID). The final shape a protein takes determines how it works in the body. The final folding of the protein is due in part to the interactions of the various amino acids, but it is also a result of the protein’s interactions with the water surrounding it. When is comes to water, amino acids can have slightly different personalities. Some are hydrophilic (they love water) and some are hydrophobic (they prefer not to interact with water). During the folding process, water forces regions of the protein with lots of hydrophobic amino acids to be tucked to the inside of the protein molecule, while regions with hydrophilic amino acids are rotated to the outside. |
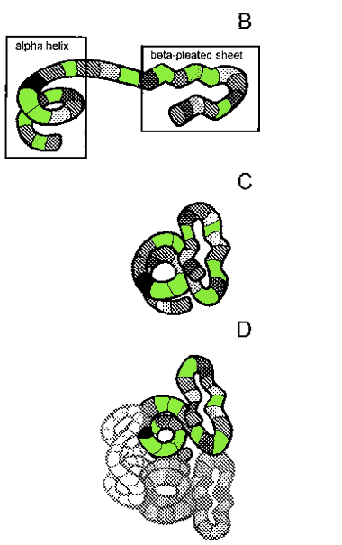
Figure 7.1 Folding of a fictitious protein showing primary (A), secondary (B), tertiary (C) and quaternary (D) structures. Amino Acids are represented in different shades of gray in the protein. See text for further explanation. |
Once it has assumed its final shape, rhodopsin is inserted into the lipid membrane of a rod cell’s disk (Figs. 3.2C and 7.3). Rhodopsin is bigger than the width of the membrane and as a result it sticks out on either side (Fig.7.3). Because of this, we say that rhodopsin is a transmembrane protein. The opsin is stable in the membrane because of the specific arrangement of the protein’s hydrophilic and hydrophobic amino acids. The inside of the membrane is composed of highly hydrophobic lipids (fats). Thus, the regions of the opsin imbedded in the membrane are also composed of primarily hydrophobic amino acids. The portions that extend outside the membrane into the watery cytoplasm of the cell consist primarily of hydrophilic amino acids.
The ability of rhodopsin to sense light depends on the small retinal molecule tucked inside the larger opsin. Retinal is a chemically-modified version of Vitamin A. Although most invertebrates can make it, vertebrates cannot and must import the raw materials. We synthesize retinal from the Beta-carotene in our diet (of which carrots are a great source). One important note before you run out and try the Bugs Bunny celebrity diet: assuming you already eat a balanced diet, chowing down on a bunch of extra carrots will not improve your vision.
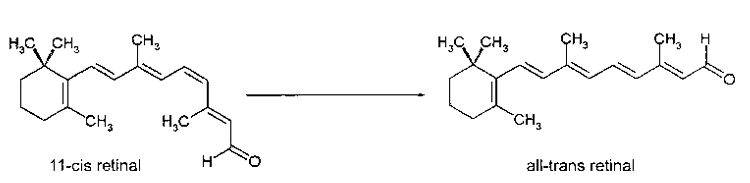
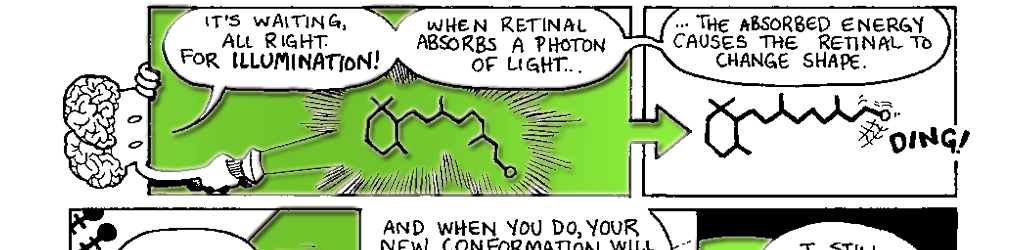
Figure 7.2 The conversion of 11-cis retinal to all-trans retinal by the absorption of a photon of light.
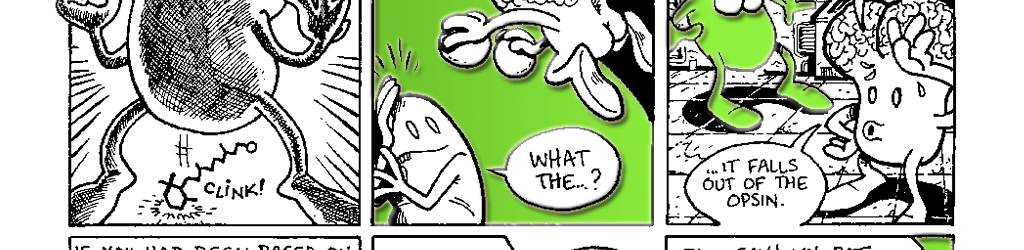
The transduction of light into a biological signal begins when the retinal molecule in rhodopsin absorbs a photon of light. The energy of the photon causes the retinal molecule to undergo a change in shape. The retinal shifts from its 11-cis retinal form (Fig. 7.2A) to its all-trans retinal form (Fig. 7.2B). Recall that the retinal is inside the opsin. When it changes shape, the retinal straightens out and pushes on the inside of the opsin. Much like a sleeping pregnant woman may assume a new position when her restless baby changes position, the opsin changes shape to accommodate retinal’s new conformation. As we mentioned earlier, a protein’s shape determines its function. When the opsin changes shape in response to the retinal its function changes. It now has the ability to interact with special proteins known as G-proteins.
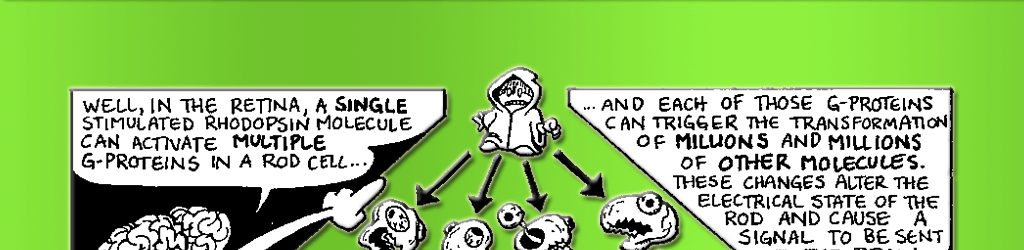
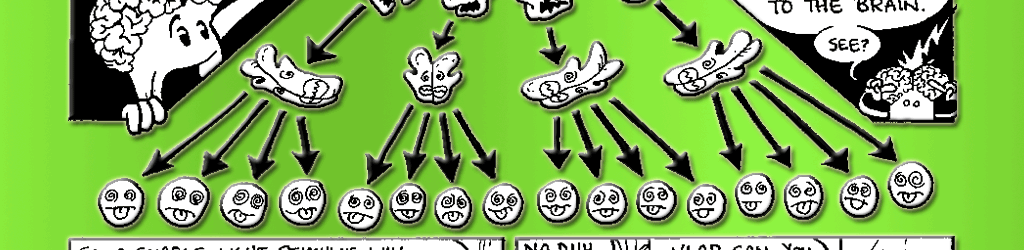
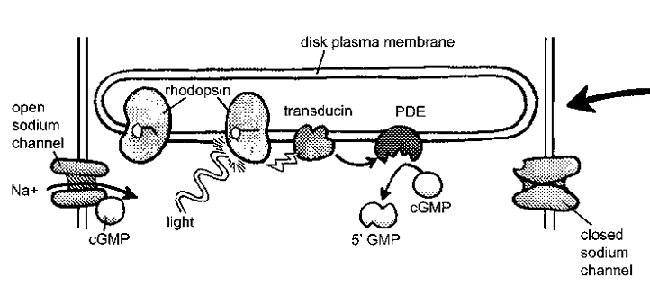 Figure 7.3 The transducin photocascade.
A photon of light stimulates
a rhodopsin molecule that
changes shape and activates
a G-protein called trandsducin.
Transducin activates phosphodiesterase
which converts cGMP to
5’GMR The loss of cGMP causes
cGMP-gated sodium channels to
close and cancels the sodium current
flowing into the rod. |
 |
How We See Color (or Keeping Your Opsins Open)The photopigments in the cones transduce light in a similar fashion. In fact, all three contain the exact same retinal molecule found in rhodopsin. So, how is it that they are tuned to different wavelengths of light? The important differences are in the opsins that surround each retinal molecule. The S, M and L cones all have opsins with slightly different primary structures than the rhodopsin opsin. The difference is only a few amino acids out of hundreds, but the effect on spectral sensitivity is profound. |
Rhodopsin clearly plays an important role in absorbing photons, but how does it tell the rest of the cell that the lights are on? It’s the messenger, but it’s also trapped in the membrane. To spread the word, it needs a second messenger molecule to carry the signal from the membrane to the rest of the cell. In the visual system, this role is played by a G-protein known as transducin. G-proteins are a special class of proteins that are activated by receptors in the cell membrane and go on to activate proteins elsewhere in the cell. Once stimulated by a single photon of light, one rhodopsin can activate approximately 500 transducin molecules. (See Fig. 7.3 for details of the following reactions.) This represents a tremendous amplification of the signal! Each of the activated 500 transducin molecules can then activate one molecule of the enzyme phosphodiesterase (PDE). But before we discuss the significance of PDE, we need to turn our attention to another aspect of this signal cascade.
The cascade of chemical reactions kicked off when a photon is absorbed eventually changes the electrical current flowing across the membrane of the rod or cone. An electrical current is defined as the flow of charged particles. When we think of electrical current flowing though a light bulb, we are talking about the flow of negatively-charged particles called electrons. In nerve cells, current is generated by the flow of charged particles called ions, like sodium (Na+), potassium (K+) and chloride (Cl ).
Ions flow across the cell membrane of rods and cones through protein channels. Protein channels are transmembrane proteins that act as gated pores in the cell membrane. These channels allow charged ions to flow into and out of the cell. When a rod’s rhodopsin is unstimulated, the sodium channels are open, which means sodium (and consequently positive electrical current) is flowing into the cell. This current, which is flowing into the cell when the lights are off, is called the dark current. This is an unusual situation. Normally, electrical current flows in a nerve when it is stimulated, not when it is NOT being stimulated.
Okay, now back to PDE. The sodium channels in the rod are held open by small molecules called cyclic GMP (or cGMP, for short). When PDE is activated by transducin, it begins turning off cGMP. When that happens, cGMP levels in the cell decline and the sodium channels close. This terminates the dark current and the rod turns off. Yes, you read that correctly. You can see things because when light hits your rods and cones, the cells turn off.
In Chapter 6 we mentioned that plants are phototrophic. They often bend toward the light to promote a process called photosynthesis. As the names suggests, photosynthesis is the process by which a plant uses the energy of a photon to synthesize something (in this case, sugar). In plants, photons are absorbed by a photopigment known as chlorophyll and the photon’s energy is channeled into a system that uses it to turn water and carbon dioxide into sugar.
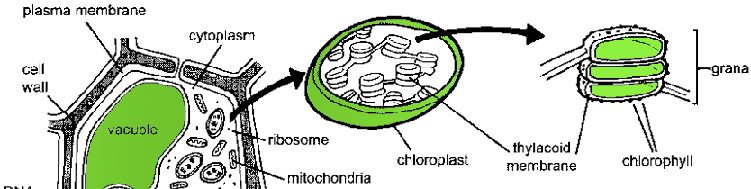
Photosynthesis takes place in a small sub-compartment of the plant cell called a chloroplast (Fig. 7.4). Inside the chloroplast is a network of membranes called the thylakoid membranes. These thylakoid membranes are arranged as stacks of disks called grana (a single disk is called a granum) and the membranes are studded with the chlorophyll pigments that absorb light. Although the plant doesn’t use the photosynthetic apparatus to “see,” this set-up is very reminiscent of how photopigments arc arranged in the rods of the retina. Pretty cool.
(I told you this so you could answer question #3).
 |
|
 |
Figure 7.4 Plant cells contain many of the same structures seen in animal cells (refer to Fig. 5.1). There are a few notable differences. Plant cells are much larger than animal cells and are supported by a rigid cell wall made of cellulose. They also contain a large fluid-filled vacuole and chloroplasts. The chloroplast is an organelle in plant cells that contains the stacks of thylacoid membrane disks. The disks bear the photopigment chlorophyll used to absorb photonic energy for photosynthesis. |