













Have you ever noticed that plants sitting on a sill will grow toward the light coming through the window? This tendency of plants to bend toward light is called phototropism. Plants exhibit phototropism because they utilize sunlight to turn water and carbon dioxide into the sugars that they (and we) need to survive. The utility of phototropism is clear. But how do plants know where the light is?
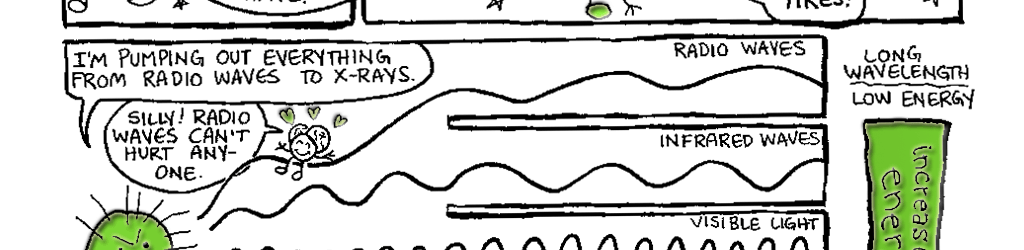
The visible light that plants and animals sense is part of a larger spectrum of electromagnetic (EM) radiation. In addition to light, this spectrum https://cdn.tumblebooks.com/syndication/excite/graphicNovels/_gn_includes several other familiar types of radiation: radio waves, microwaves, ultraviolet radiation, X-rays and gamma rays. As Figure 6.1 demonstrates, these forms of radiation exist in a continuum, one blending into the next. So, what distinguishes one type of radiation from another? Why do you need to wear a heavy lead apron when getting an X-ray, but you can be bathed in radio waves all day long without similar protection? To determine what makes them different, we first need to consider some of the fundamental properties all forms of electromagnetic radiation share. Let’s begin with a question about the nature of electromagnetic radiation: Does it travel as waves or particles? The answer is a resounding, “It depends!”
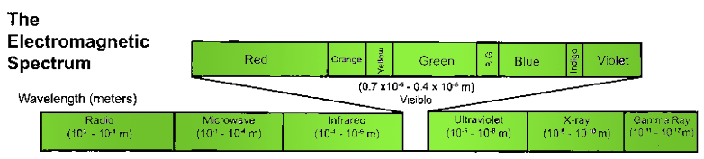
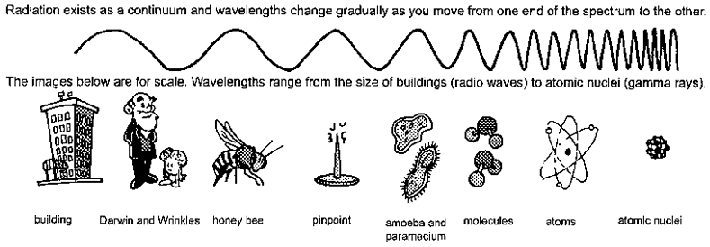
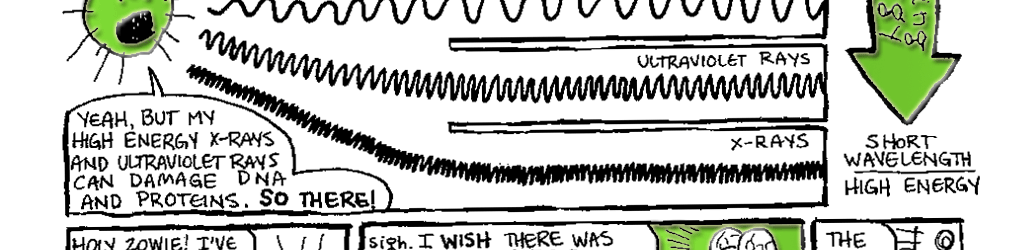
Figure 6.1 The Electromagnetic spectrum. The electromagnetic spectrum comprises a continuum of different types of radiation. At one end are low-energy radio waves. Radio waves are about as long as a building is tall. High energy gamma rays sit at the opposite end of the spectrum and have wavelengths on the scale of atomic nuclei. This figure was modified and redrawn with permission from a diagram available at My NASA Data, (mynasadata.larc.nasa.gov).
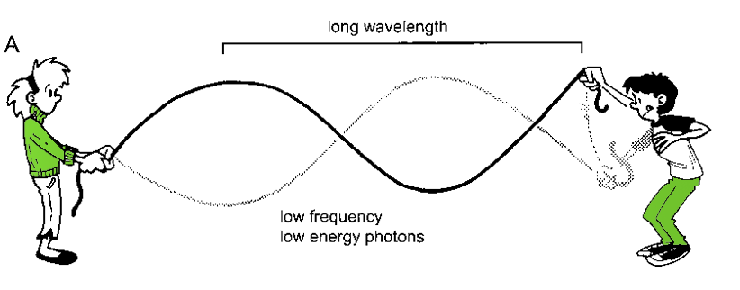
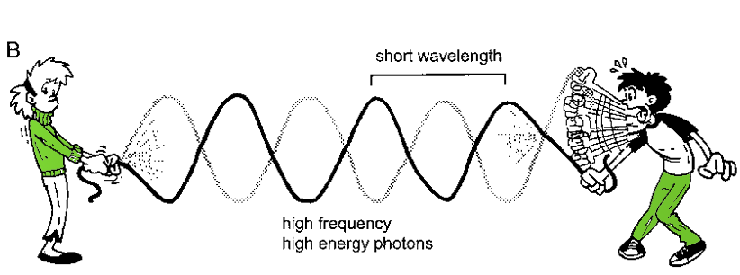
Figure 6.2. The jump rope/radiation analogy. A) Low energy waves have a long wavelength and low frequency while B) high energy waves have short wavelengths and higher frequencies.
Does light (and other EM radiation) move like a particle or a wave? This conundrum, known as the Wave/Particle duality, is one of the great puzzles of physics. When you run certain types of experiments, light behaves like a wave. To visualize waves, imagine yourself and a friend holding the ends of a long rope. While your friend holds her end still, you raise and lower yours. If you do this slowly, you create a single long wave in the rope (Fig. 6.2A). However, if you pump your arm rapidly, you generate several smaller waves (Fig. 6.2B). In the second case, you have shortened the wavelength and increased the frequency of the waves. The wavelength is the distance between the peaks of two successive waves. X-rays have shorter wavelengths than radio waves and, as a result, they also have a higher frequency, or number of waves over a given period of time.
When viewed through a different set of experiments, light behaves like it is composed of little packets of energy called photons. These photons are discrete particles that can collide with and be absorbed by objects.
So, why are X-rays potentially hazardous to your health while radio waves are fairly benign? The answer is that X-ray photons have more energy. Imagine the difference between a suitor gentlytossing a pebble at your window or firing the same pebble from a bazooka. The latter pebble has more energy and consequently can do more damage. Likewise, when your cells are bombarded by Xray photons, the DNA and proteins are getting hit with high-energy photons that have the potential to do a lot of damage.
In our discussion of light and vision, we will move between the wave and particle descriptions of light. When discussing light and color, we will talk about wavelengths and frequencies of light. When we later discuss how light is absorbed in the eye, we will talk about how photopigments absorb photons of light. For our purposes, we can reconcile the Wave/Particle duality by saying that a photon’s energy is directly proportional to its frequency. In other words, the more energy a photon of a particular type of radiation has, the higher its frequency (Fig. 6.1). Thus, X-ray photons are more energetic than radio photons, and X-ray waves have a higher frequency than radio waves.
Visible light sits sandwiched between X-rays and radio waves on the electromagnetic spectrum. It has more energy than radio waves but less than X-rays. Visible light can interact with molecules in our cells, but unlike X-rays, it typically can do so without doing damage (although, if you make it bright enough, it can still blind you, so no staring at the sun). Although it represents only a sliver of the electromagnetic spectrum, visible light can be further subdivided by passing it through a prism. It emerges as a spectrum of colors (or a rainbow, if you prefer). The different colors in the spectrum represent visible light of slightly different energies. Violet light is the most energetic with a wavelength of approximately 400 nanometers (nm; one billionth of a meter), and red light falls at the less energetic end of the spectrum at 700 nm.
At the age of 72, Charles Darwin conducted a series of experiments with his son Francis on the movement of plants (eventually published in The Power o f Movement in Plants, 1880). In one set of experiments, they examined phototropism in reed canary grass. They grew grass next to several different colored lights and found that only shoots growing next to the blue light exhibited phototropism. The other colored lights had no effect on the grass shoots. Thus, the plants weren’t just sensing light, they were sensing a particular part of the light.
We now know that plants, just like animals’ eyes, contain lightabsorbing molecules known as photopignients. There are several different types of photopigments found in nature, and each type tends to be tuned to a specific range of the visible-light spectrum. In the Darwins’ experiment, phototropism in reed canary grass is triggered when photopigments absorb blue wavelengths of light (450 nm 500 nm). But, if photopigments only respond to specific chunks of the rainbow, how many different types do we need in order to see the breathtakingly subtle hues of a sunset? Turns out, for humans, the magic number is three.
Photopigments allow plants, animals and some microorganisms to perceive and respond to light. In the case of plants and animals like flatwomis, simple sets of light sensitive cells are adequate. These organisms just need to know if the lights are on or off. But, as we have seen, some animals have evolved eye structures around the cells that contain their photopigments. Eyes manipulate the light to varying degrees so that an image of the environment is formed. Depending on the environment and lifestyle of a species, that image may be monochromatic (black, white and shades of gray) or very colorful. Nocturnal animals, for example, tend to possess a single type of photopigment and have monochromatic vision.
Humans also have monochromatic vision at night. Everything appears in shades of gray as we stumble through the dark on our way to the bathroom. The photopigment at work in our eyes at this time is called rhodopsin, and it is very sensitive to low levels of light. But, if you turn on the bathroom lights, the rhodopsin becomes overwhelmed by the brightness and shuts down completely. So how is it that you can still see to check if the toilet seat is down?
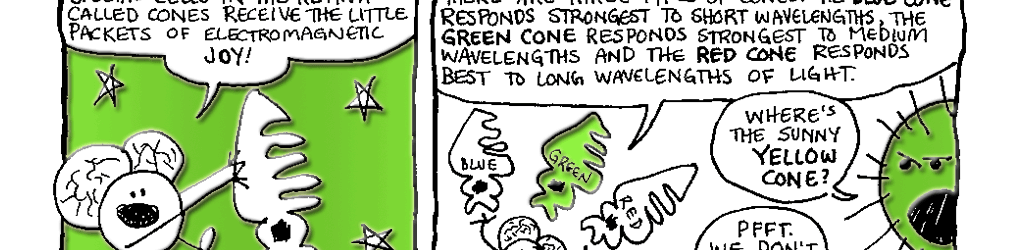
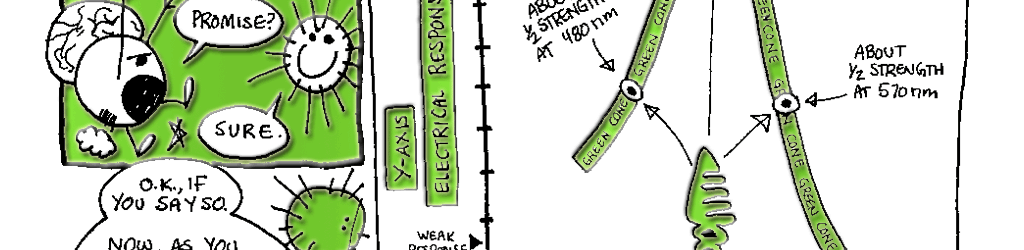
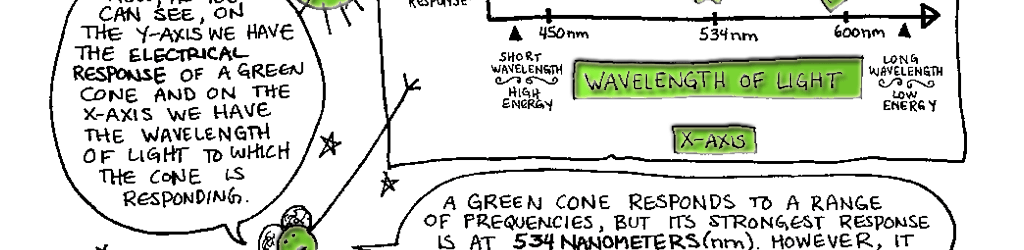
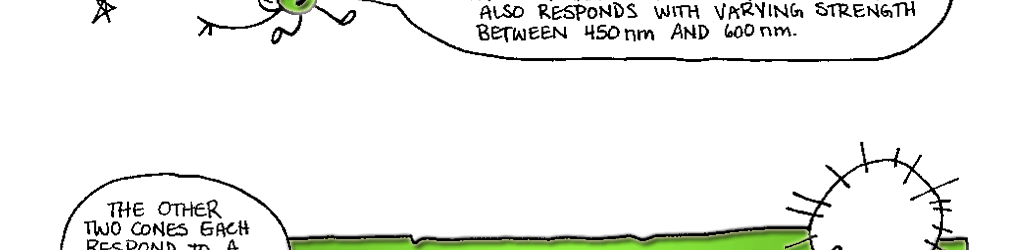
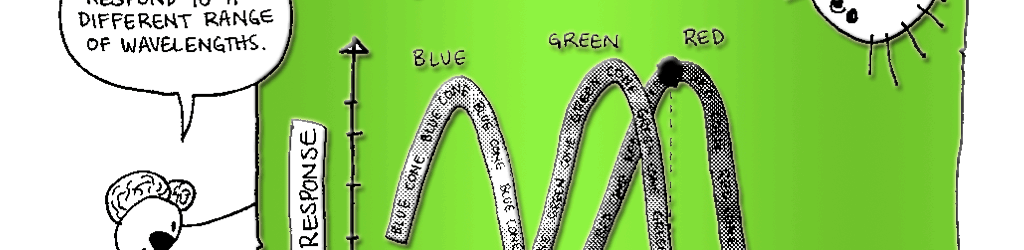
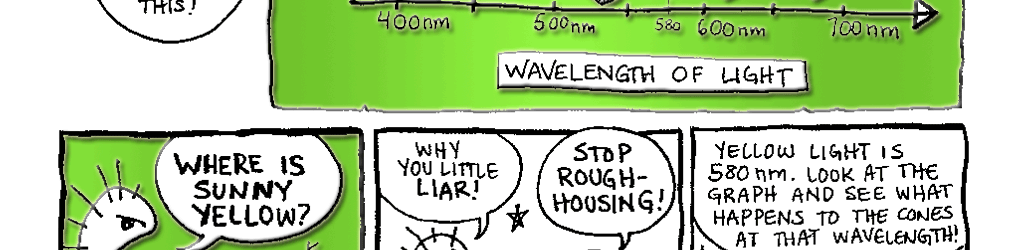
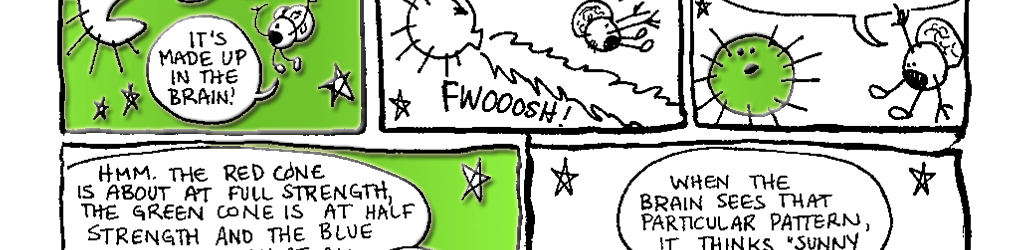
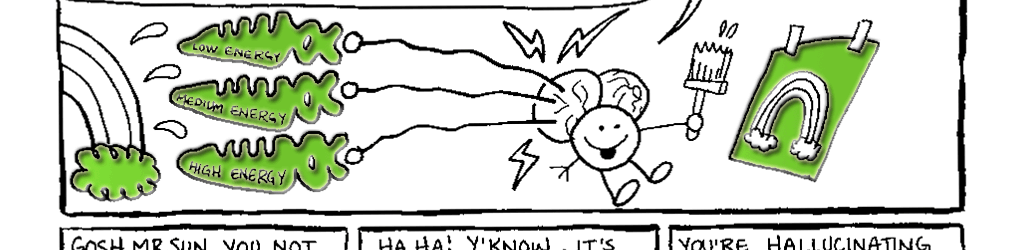
Day vision is handled by a trio of photopigments. These pigments are distributed in three different types of cone cells in the retina: the S-Cone preferentially responds to the short wavelengths of light at the blue end of the spectrum, the M-cone responds to green light of medium wavelength and the L-cone responds to long wavelengths of light at the red end of the spectrum. But we live in a world painted in far subtler hues than just blue, green and red. How do three photopigments generate the myriad colors we see everyday? This is possible because 1) the photopigments in each cone are tuned to a range of wavelengths and 2) their response ranges overlap (see the graph on pg. 76). Thus, light of any given wavelength may stimulate more than one type of cone. For example, light with a wavelength of 525 nm will activate all three cones. The M-cone will be strongly activated, the S-cone will be weakly activated and the L-cone will be activated at some intermediate value. When the brain receives the information from the cones, it pays attention to the strengths of the three signals relative to each other. When it registers the relative activity levels described above, it decodes the signal as the color greenish-yellow.
The implications of this system are clear: Color is all in your head. The writer Flannery O'Connor wrote, "The beginning of human knowledge is through the senses.... " It is absolutely true that what we perceive as reality is in large part determined by the nature 82 of our sensory systems. And, in turn, the nature of our sensory systems is a product of our evolutionary history.
Other species have different needs and thus different sensory systems. For example, honey bees have a visual spectrum that is shifted relative to ours. They do not see red as we do, but they do see the ultraviolet colors of the flowers they pollinate. (We need a black light to do that.) In fact, there is a whole world of sensory stimuli that are beyond our capacity to perceive. Plants sense chemicals in the soil, sharks sense the minute electrical signals of their prey’s nervous system and sea turtles navigate using magnetic fields. It makes us reconsider what we define as reality and brings to mind the words of the astronomer Sir Arthur Stanley Eddington, “Not only is the universe stranger than we imagine, it is stranger than we can imagine.